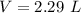Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

You know the right answer?
zinc metal and hydrochloric acid react together according to the following equation 2HCl (aq)+ Zn(s)...
Questions

Social Studies, 26.11.2021 22:00


Business, 26.11.2021 22:00



Mathematics, 26.11.2021 22:00

Business, 26.11.2021 22:00






Geography, 26.11.2021 22:00

History, 26.11.2021 22:00

Social Studies, 26.11.2021 22:00

Mathematics, 26.11.2021 22:00





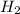 would be Zn. So, we have to follow a few steps:
would be Zn. So, we have to follow a few steps: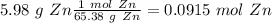

 , so:
, so: