
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and in a coffee-cup calorimeter. In one measurement, qrxn=−13.3qrxn=−13.3 kJkJ in the other qrxn=−11.9qrxn=−11.9 kJkJ. Which value was obtained in the bomb calorimeter? (Assume that the reaction has a positive ΔVΔV in the coffee-cup calorimeter.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and...
Questions

Chemistry, 04.11.2020 03:30

Mathematics, 04.11.2020 03:30

English, 04.11.2020 03:30

Spanish, 04.11.2020 03:30

Biology, 04.11.2020 03:30

History, 04.11.2020 03:30


Mathematics, 04.11.2020 03:30




Mathematics, 04.11.2020 03:30



English, 04.11.2020 03:30



History, 04.11.2020 03:30


Mathematics, 04.11.2020 03:30

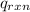 = -13.3 KJ belongs to the bomb calorimeter
= -13.3 KJ belongs to the bomb calorimeter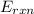 of the reaction, the work of the reaction
of the reaction, the work of the reaction 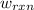 and the heat of the reaction
and the heat of the reaction 

