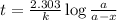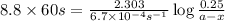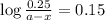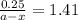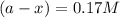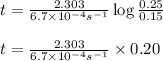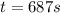Chemistry, 26.06.2020 15:01 allimaycatp8qgaq
The conversation of cyclopropane to propene in the gas phase is a first order reaction with a rate constant of 6.7x10-⁴s-¹. a) if the initial concentration of cyclopropane was 0.25M , what is the concentration after 8.8min?b) how long (in min) will it take for the concentration of cyclopropane to decrease from 0.25M to 0.15M?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1



Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1
You know the right answer?
The conversation of cyclopropane to propene in the gas phase is a first order reaction with a rate c...
Questions

Chemistry, 08.11.2021 08:50

French, 08.11.2021 08:50


Mathematics, 08.11.2021 08:50

Chemistry, 08.11.2021 08:50


Social Studies, 08.11.2021 08:50


English, 08.11.2021 08:50



Chemistry, 08.11.2021 08:50


Computers and Technology, 08.11.2021 08:50



Mathematics, 08.11.2021 08:50

Mathematics, 08.11.2021 08:50