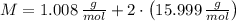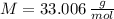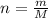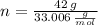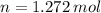Chemistry, 23.06.2020 16:01 leahstubbs
How many moles of \ce{H2O}HX 2 O will be produced from 42.0 \text{ g}42.0 g42, point, 0, start text, space, g, end text of \ce{H2O2}HX 2 OX 2 ?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3
You know the right answer?
How many moles of \ce{H2O}HX 2 O will be produced from 42.0 \text{ g}42.0 g42, point, 0, start text...
Questions


Mathematics, 29.03.2020 03:47



History, 29.03.2020 03:47

Biology, 29.03.2020 03:47



Mathematics, 29.03.2020 03:47

Mathematics, 29.03.2020 03:47

Geography, 29.03.2020 03:47




Social Studies, 29.03.2020 03:47

Biology, 29.03.2020 03:47

Mathematics, 29.03.2020 03:48



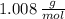 and
and 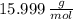 , respectively. A mole is the ratio of current mass of water to molecular weight of water, the latter one is now calculated before computing the amount of moles of water:
, respectively. A mole is the ratio of current mass of water to molecular weight of water, the latter one is now calculated before computing the amount of moles of water: