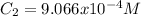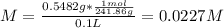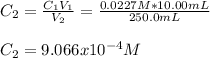Chemistry, 20.06.2020 18:57 CuteDoggo1828
A solution is prepared by dissolving 0.5482 grams of iron(III) nitrate in enough water to make 100.0 mL of solution. A 10.00 mL aliquot (portion) of this solution is then diluted to a final volume of 250.0 mL. What is the concentration of Fe3 ions (M) in the final solution

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2


Chemistry, 23.06.2019 09:30
The earth's surface is (science) a: studied using seismic waves b: constantly changing over time c: only studied indirectly d: the same today as million of years
Answers: 1
You know the right answer?
A solution is prepared by dissolving 0.5482 grams of iron(III) nitrate in enough water to make 100.0...
Questions

Computers and Technology, 30.04.2021 17:30



World Languages, 30.04.2021 17:30


History, 30.04.2021 17:30




History, 30.04.2021 17:30



English, 30.04.2021 17:30

English, 30.04.2021 17:30


Biology, 30.04.2021 17:30

Mathematics, 30.04.2021 17:30



Mathematics, 30.04.2021 17:30