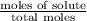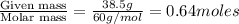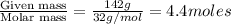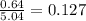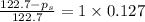Chemistry, 13.06.2020 23:57 reginaldlegette
Calculate the vapor pressure (in torr) at 298 K in a solution prepared by dissolving 38.5 g of the non-volatile non-electrolye urea {CO(NH2)2} in 142 g of methanol. The vapor pressure of methanol at 298 K is 122.7 torr. Give your answer to 2 decimal places.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 21:30
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
Calculate the vapor pressure (in torr) at 298 K in a solution prepared by dissolving 38.5 g of the n...
Questions




Chemistry, 09.12.2020 22:10

Mathematics, 09.12.2020 22:10

Mathematics, 09.12.2020 22:10


Mathematics, 09.12.2020 22:10


Chemistry, 09.12.2020 22:10

Mathematics, 09.12.2020 22:10



English, 09.12.2020 22:10

Mathematics, 09.12.2020 22:10


Mathematics, 09.12.2020 22:10



Mathematics, 09.12.2020 22:10


 = relative lowering in vapor pressure
= relative lowering in vapor pressure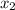 = mole fraction of solute =
= mole fraction of solute =