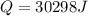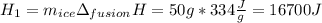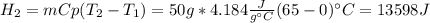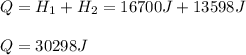Chemistry, 13.06.2020 09:57 xbeatdroperzx
How many joules of heat are needed to melt 50 g of ice at 0°C and then warm the liquid to 65°C? heat of fusion of ice = 334 J/g specific heat of water = 4.184 J/g°C specific heat of ice = 2.03 J/g°C heat of vaporization of water = 2260J/g

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
How many joules of heat are needed to melt 50 g of ice at 0°C and then warm the liquid to 65°C? heat...
Questions

Mathematics, 25.10.2021 14:00


History, 25.10.2021 14:00


Mathematics, 25.10.2021 14:00


Mathematics, 25.10.2021 14:00



Mathematics, 25.10.2021 14:00






Mathematics, 25.10.2021 14:00


History, 25.10.2021 14:00