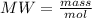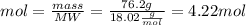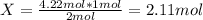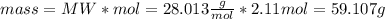Chemistry, 12.06.2020 01:57 alshaibanihassan10
The substances nitrogen monoxide and hydrogen gas react to form nitrogen gas and water. Unbalanced equation: NO (g) + H2 (g) N2 (g) + H2O (l) In one reaction, 76.2 g of H2O is produced. What amount (in mol) of H2 was consumed? What mass (in grams) of N2 is produced?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

You know the right answer?
The substances nitrogen monoxide and hydrogen gas react to form nitrogen gas and water. Unbalanced e...
Questions


Mathematics, 06.11.2020 18:50



Biology, 06.11.2020 18:50


Mathematics, 06.11.2020 18:50

History, 06.11.2020 18:50


Mathematics, 06.11.2020 18:50





Mathematics, 06.11.2020 18:50

Mathematics, 06.11.2020 18:50


Computers and Technology, 06.11.2020 18:50

Chemistry, 06.11.2020 18:50

Physics, 06.11.2020 18:50