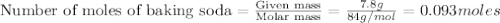Chemistry, 10.06.2020 06:57 smithsa10630
1.)A strong acid solution requires 3.2 grams of sulfuric acid (H2SO4). How many molecules of sulfuric acid are in the solution? 2.) While measuring out the sulfuric acid you accidentally spilled some of it! Before trying to clean it up you put some baking soda (NaHCO3) on to it neutralize it. If you scatter 7.8 g of baking soda on the acid how many moles of baking soda have you used?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3
You know the right answer?
1.)A strong acid solution requires 3.2 grams of sulfuric acid (H2SO4). How many molecules of sulfuri...
Questions

Business, 21.04.2020 23:41



Mathematics, 21.04.2020 23:41



Mathematics, 21.04.2020 23:41





Mathematics, 21.04.2020 23:41




Chemistry, 21.04.2020 23:42

Mathematics, 21.04.2020 23:42



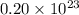 molecules of sulfuric acid in the solution.
molecules of sulfuric acid in the solution.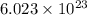 of particles.
of particles.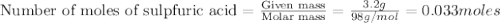
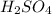 contains =
contains = 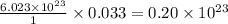 molecules of sulfuric acid
molecules of sulfuric acid