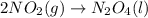Chemistry, 07.06.2020 13:57 Spence8900
PLZ HELP For the reaction: 2NO2(g) → N2O4(l),
the ΔH of the reactants, two moles of NO2 (g), is + 66 kJ/mol,
and the ΔH of the products, N2O4 (l), is -20 kJ/mol.
Which of the following shows the ΔH (change in enthalpy) for the reaction as a whole?
Question 8 options:
ΔrxnH =(- 20 kJ/mol) / (+66 kJ/mol)
ΔrxnH = (+66 kJ/mol) + (- 20 kJ/mol)
ΔrxnH =(-20 kJ/mol) - (+ 66 kJ/mol)
ΔrxnH =(+66 kJ/mol) - (- 20 kJ/mol)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1


Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
PLZ HELP For the reaction: 2NO2(g) → N2O4(l),
the ΔH of the reactants, two moles of NO2 (g), is + 6...
Questions


Mathematics, 11.11.2019 18:31


English, 11.11.2019 18:31

Mathematics, 11.11.2019 18:31

History, 11.11.2019 18:31




Mathematics, 11.11.2019 18:31


Mathematics, 11.11.2019 18:31

Biology, 11.11.2019 18:31

English, 11.11.2019 18:31

Arts, 11.11.2019 18:31

Mathematics, 11.11.2019 18:31



Biology, 11.11.2019 18:31

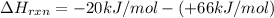
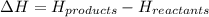
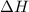 = enthalpy change = ?
= enthalpy change = ? = enthalpy of products
= enthalpy of products 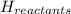 = enthalpy of reactants
= enthalpy of reactants