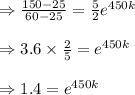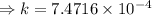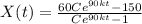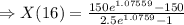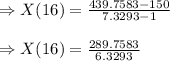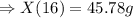Chemistry, 07.06.2020 05:58 hailiemanuel930
Two chemicals A and B are combined to form a chemical C. The rate, or velocity, of the reaction is proportional to the product of the instantaneous amounts of A and B not converted to chemical C. Initially, there are 40 grams of A and 50 grams of B, and for each gram of B, 2 grams of A is used. It is observed that 25 grams of C is formed in 8 minutes. How much (in grams) is formed in 16 minutes

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2

You know the right answer?
Two chemicals A and B are combined to form a chemical C. The rate, or velocity, of the reaction is p...
Questions

Mathematics, 11.02.2020 20:00


Health, 11.02.2020 20:00

Mathematics, 11.02.2020 20:00

History, 11.02.2020 20:00







Mathematics, 11.02.2020 20:00






Mathematics, 11.02.2020 20:00


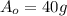 and
and 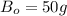
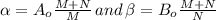
 part of the chemical C we will need 2 part of A and one part of B.
part of the chemical C we will need 2 part of A and one part of B.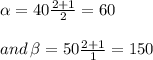
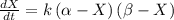
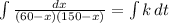
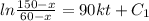
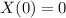 ,
,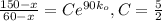
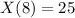 and solving for the value of 'k'
and solving for the value of 'k'