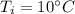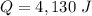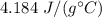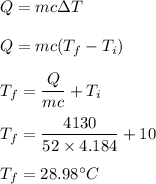Chemistry, 06.06.2020 04:00 zaniyastubbs9
A 52-gram sample of water that has an initial temperature of 10.0 °C absorbs 4,130 joules. If the specific heat of water is 4.184 J/(g °C), what is the final temperature of the water?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 08:30
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1

Chemistry, 23.06.2019 12:30
Choose one literary selection from this semester in which you think the setting has a great impact on the work. in a full paragraph name the work, describe the setting, and explain why it is so important to the overall story or poem.
Answers: 1
You know the right answer?
A 52-gram sample of water that has an initial temperature of 10.0 °C absorbs 4,130 joules. If the sp...
Questions

Physics, 09.04.2021 16:40

Arts, 09.04.2021 16:40


Biology, 09.04.2021 16:40

Computers and Technology, 09.04.2021 16:40

English, 09.04.2021 16:40

Social Studies, 09.04.2021 16:40




Mathematics, 09.04.2021 16:40







English, 09.04.2021 16:40

Mathematics, 09.04.2021 16:40