
Chemistry, 06.06.2020 00:03 yuppcami9929
Br2(g) + 3 F2(g) ⇄ 2 BrF3(g) Kp = 5.4 × 108 0.30 atm of Br2 and 0.60 atm of F2 are placed in a 3.0 L container and the system is allowed to reach equilibrium. Calculate the pressureof Br2 at equilibrium.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
Br2(g) + 3 F2(g) ⇄ 2 BrF3(g) Kp = 5.4 × 108 0.30 atm of Br2 and 0.60 atm of F2 are placed in a 3.0 L...
Questions

History, 17.11.2019 00:31


Biology, 17.11.2019 00:31


Mathematics, 17.11.2019 00:31

Geography, 17.11.2019 00:31

Chemistry, 17.11.2019 00:31

Mathematics, 17.11.2019 00:31


Mathematics, 17.11.2019 00:31

Mathematics, 17.11.2019 00:31


Mathematics, 17.11.2019 00:31

English, 17.11.2019 00:31

Computers and Technology, 17.11.2019 00:31



Mathematics, 17.11.2019 00:31

Social Studies, 17.11.2019 00:31

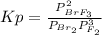 = 5.4x10⁸
= 5.4x10⁸

