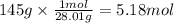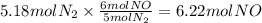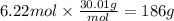Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Dying the folding patterns of protein molecules can microbiologists better understand cellular processes as well as some diseases, such as alzheimer’s, that are caused by proteins that have misfolded. the folding of these complicated molecules can be simulated on computers, but it takes a lot of processor power and time for even expensive supercomputers to do this. a group of researchers at stanford university developed software that can be used to distribute the processing of data to anyone who is willing to donate time on their idle personal computers. as a result, the researchers have been able to achieve protein-folding simulations that are far better than those other computing methods have done. which statement best describes the work of these researchers? the work is not scientific because the data are not processed in one location. the work is not scientific because the simulations are not reproducible. the researchers applied creativity to solve a problem in running an experiment. the researchers used only well-established scientific techniques.
Answers: 3

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
How many grams of NO are required to produce 145 g of N2 in the following reaction?
4NH3(g) + 6NO(g...
Questions