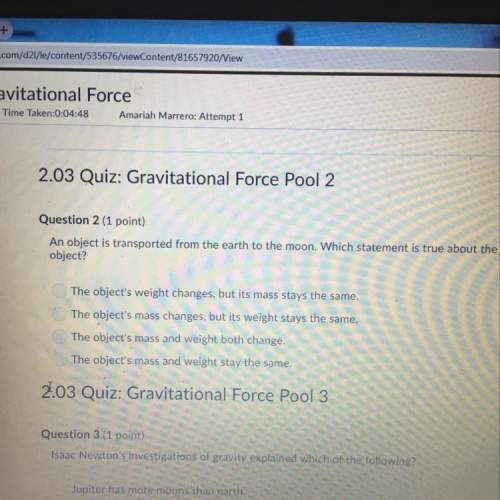
CHEM will give all pointss
PLEASE HELP!! REALLY STUCK ON THIS QUESTION AND I HAVE WRITERS BLOCK RIGHT NOW
4. A calorimeter contains 500 g of water at 25°C. You place a cold pack containing 100 g of crystalline ammonium nitrate inside the calorimeter. When the ammonium nitrate finishes dissolving, the temperature of the water inside the calorimeter is 9.3°C. The specific heat of water is 4.18 J/g–°C. What is the enthalpy of fusion (ΔHf) of the ammonium nitrate? (Show your work.) Where necessary, use q = mHf.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
CHEM will give all pointss
PLEASE HELP!! REALLY STUCK ON THIS QUESTION AND I HAVE WRITERS BLOCK RIG...
Questions

History, 22.07.2019 18:30


Mathematics, 22.07.2019 18:30







English, 22.07.2019 18:30



Social Studies, 22.07.2019 18:30






Biology, 22.07.2019 18:30