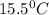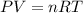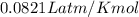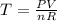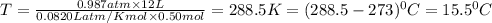Chemistry, 03.06.2020 09:57 muravyevaarina
Calculate the temperature of a 0.50 mol sample of a gas at 0.987 atm and a volume of 12 L.
-7 C
11 C
15.5 C
288 C

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Anurse practitioner prepares an injection of promethazine, an antihistamine used to treat allergic rhinitis. if the stock bottle is labeled 25 mg/ml and the order is a dose of 11.0 mg , how many milliliters will the nurse draw up in the syringe?
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1

You know the right answer?
Calculate the temperature of a 0.50 mol sample of a gas at 0.987 atm and a volume of 12 L.
Questions


Physics, 10.07.2019 22:50


Health, 10.07.2019 22:50

English, 10.07.2019 22:50

Physics, 10.07.2019 22:50

English, 10.07.2019 22:50



Computers and Technology, 10.07.2019 22:50





History, 10.07.2019 22:50




History, 10.07.2019 22:50