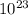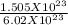A sample of sugar (C12H22O11) contains
1.505 × 1023 molecules of sugar. How many moles of suga...

Chemistry, 02.06.2020 22:00 chiarag305
A sample of sugar (C12H22O11) contains
1.505 × 1023 molecules of sugar. How many moles of sugar are present in the sample? Answer without doing any calculations.
0.25 mol
0.50 mol
1.00 mol
2.50 mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
Questions

Chemistry, 22.04.2021 23:20


Biology, 22.04.2021 23:20






Mathematics, 22.04.2021 23:20


Mathematics, 22.04.2021 23:20

Mathematics, 22.04.2021 23:20

Advanced Placement (AP), 22.04.2021 23:20



History, 22.04.2021 23:20

Mathematics, 22.04.2021 23:20