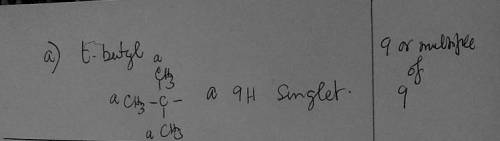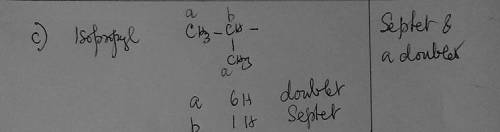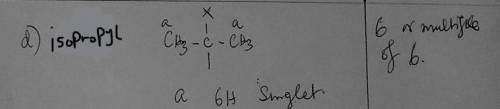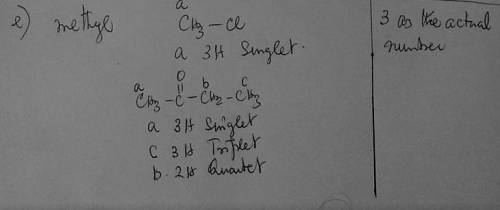Chemistry, 02.06.2020 07:59 he0gaubong
There are certain trends with which you should become very familar (recognizing these trends will save you time!) This part of the question is dedicated to that task.
a) Whenever you see 9 or a multiple of 9 in the integration ratio, which group should you first consider for being responsible for that signal.
b) Whenever you see a quartet and triplet on a spectrum, which group should you first consider for being responsible for those signals?
c) Whenever you see septet and a doublet on a spectrum, which group should you first consider for being responsible for those signals?
d) Whenever you see 6 or a multiple of 6 in the integration ratio, which group should you first consider for being responsible for that signal
e) Whenever you see 3 as the actual number of protons for a given signal, which group should you first consider for being responsible for that signal

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
There are certain trends with which you should become very familar (recognizing these trends will sa...
Questions


English, 15.11.2019 03:31



English, 15.11.2019 03:31


English, 15.11.2019 03:31


Mathematics, 15.11.2019 03:31




Mathematics, 15.11.2019 03:31

Mathematics, 15.11.2019 03:31

Mathematics, 15.11.2019 03:31



Mathematics, 15.11.2019 03:31

Mathematics, 15.11.2019 03:31