
Chemistry, 29.05.2020 22:58 yulimariu27
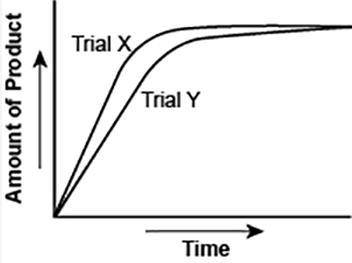
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a lower concentration of the reactant?
A) Trial X, because the final volume of product formed is lower than Trial Y.
B) Trial X, because this reaction was initially fast and later stopped completely.
C) Trial Y, because the reaction was initially slow and later stopped completely.
D) Trial Y, because the volume of product formed per unit time is lower than Trial X.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions



Mathematics, 06.01.2020 15:31

Health, 06.01.2020 15:31

Chemistry, 06.01.2020 15:31



History, 06.01.2020 15:31


Health, 06.01.2020 15:31




English, 06.01.2020 15:31








