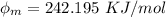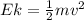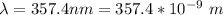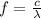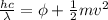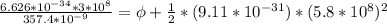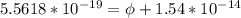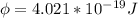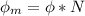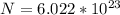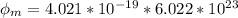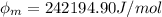Chemistry, 29.05.2020 09:57 lilyrockstarmag
In order to comply with the requirement that energy be conserved, Einstein showed in the photoelectric effect that the energy of a photon (h) absorbed by a metal is the sum of the work function (), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron: h = + Ek. When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is (in kJ/mol) of potassium?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
In order to comply with the requirement that energy be conserved, Einstein showed in the photoelectr...
Questions

Physics, 18.04.2021 19:30

Mathematics, 18.04.2021 19:30



Chemistry, 18.04.2021 19:30


Mathematics, 18.04.2021 19:30


Mathematics, 18.04.2021 19:30

Mathematics, 18.04.2021 19:30


Mathematics, 18.04.2021 19:30

Chemistry, 18.04.2021 19:30


History, 18.04.2021 19:30


Advanced Placement (AP), 18.04.2021 19:30

History, 18.04.2021 19:30

Mathematics, 18.04.2021 19:30

Chemistry, 18.04.2021 19:30

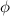 ), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron:
), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron: 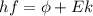 . When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is work function (in kJ/mol) of potassium?
. When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is work function (in kJ/mol) of potassium?