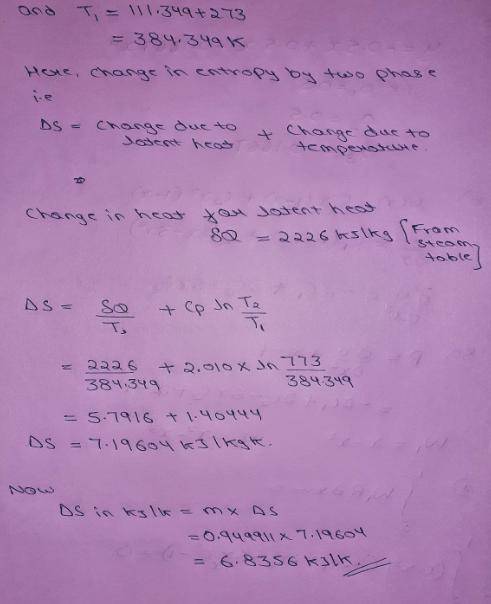Chemistry, 28.05.2020 19:01 donaldplawlerp5cctt
An insulated piston-cylinder device contains 1 L of saturated liquid water at pressure of 150 kPa. An electric resistance heater inside the cylinder is now turned on, and 2869.47 kJ of heat is transferred to the water. The inside H2O pressure maintains constant at 150 kPa during the process. Determine: the entropy change of the water during this heating process, in kJ/K.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Litmus paper is made from water-soluble dyes which are extracted from lichens. this paper is used as an acid-base indicator. which of these common household substances would turn blue litmus paper red? a) bleach b) lye c) soap d) vinegar
Answers: 3

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1
You know the right answer?
An insulated piston-cylinder device contains 1 L of saturated liquid water at pressure of 150 kPa. A...
Questions

History, 22.07.2019 04:21


Chemistry, 22.07.2019 04:21

Biology, 22.07.2019 04:21

Chemistry, 22.07.2019 04:21

History, 22.07.2019 04:21

History, 22.07.2019 04:21







Social Studies, 22.07.2019 04:21

Mathematics, 22.07.2019 04:21