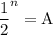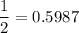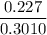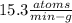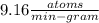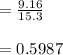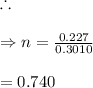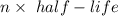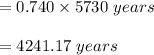In living organisms, C-14 atoms disintegrate at a rate of 15.3 atoms per minute per gram of carbon. A charcoal sample from an archaeological site has a C-14 disintegration rate of 9.16 atoms per minute per gram of carbon. Estimate the age of this sample in years. The half-life of C-14 is 5730 years. (enter only the number of years in standard notation, not the unit years)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2
You know the right answer?
In living organisms, C-14 atoms disintegrate at a rate of 15.3 atoms per minute per gram of carbon....
Questions

English, 12.12.2021 19:30


Social Studies, 12.12.2021 19:30


History, 12.12.2021 19:30

History, 12.12.2021 19:30



Mathematics, 12.12.2021 19:30

English, 12.12.2021 19:30



Physics, 12.12.2021 19:30


Mathematics, 12.12.2021 19:30

Mathematics, 12.12.2021 19:30


History, 12.12.2021 19:30


English, 12.12.2021 19:30

 = 0.5987
= 0.5987