C) Record the balanced chemical equation on the Student Worksheet.
Step 3: Determine the amoun...

Chemistry, 27.05.2020 08:58 destiny3200
C) Record the balanced chemical equation on the Student Worksheet.
Step 3: Determine the amount of energy change in the reaction.
a) Use the table of enthalpy values (Table A) provided in the Student Worksheet to locate the
enthalpy of formation (AH) for each reactant and each product. Record these values along with
the reactants and products in Table B of the Student Worksheet.
b) Determine the total enthalpy the reactants and the total enthalpy of the products. Record these
values in Table C of the Student Worksheet.
reactants)
c) Use the following formula to find the net change in enthalpy for the reaction and to determine
whether the reaction is endothermic or exothermic.
AH wen - (AH, products) -(AH
Record your answers in Table D.
Step 4: Model the energy change in the reaction.
a) Create an energy graph that illustrates the energy change in the reaction.
b) Construct your graph on a blank sheet of paper. Be sure to label the axes, provide a title, and
identify the reactants and products on the graph.
Step 5: Explain the energy change in the reaction.
a) Create a new blank document. Type your name at the top.
b) Write a few paragraphs describing the chemical reaction and explaining the energy change in
the reaction. Your document should
Did anyone do this project? And if so can you help me with these steps??

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Questions


English, 28.10.2020 19:20

Biology, 28.10.2020 19:20

History, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20

Arts, 28.10.2020 19:20


Law, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20


English, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20


Mathematics, 28.10.2020 19:20

Chemistry, 28.10.2020 19:20

Arts, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20

English, 28.10.2020 19:20

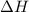 is positive then it means heat is absorbed by the system. And when
is positive then it means heat is absorbed by the system. And when 

