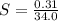Chemistry, 23.05.2020 16:59 milkshakegrande101
A geochemist in the field takes a 34.0 mL sample of water from a rock pool lined with crystals of a certain mineral compound X. He notes the temperature of the pool, 22.0 °C, and caps the sample carefully. Back in the lab, the geochemist first dilutes the sample with distilled water to 750.0 mL. Then he filters it and evaporates all the water under vacuum. Crystals of X are left behind. The researcher washes, dries, and weighs the crystals. They weigh 0.31 g.
Using only the information above, can you calculate yes the solubility of X in the water at 17.0 °C? If yes, calculate it. Be sure your answer has a unit symbol and 3 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
You know the right answer?
A geochemist in the field takes a 34.0 mL sample of water from a rock pool lined with crystals of a...
Questions

Chemistry, 17.03.2021 23:50

English, 17.03.2021 23:50



Chemistry, 17.03.2021 23:50



Mathematics, 17.03.2021 23:50

Physics, 17.03.2021 23:50



Biology, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50





Engineering, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

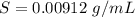
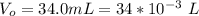
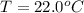
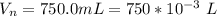
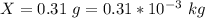
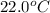 can be mathematically evaluate as
can be mathematically evaluate as