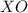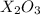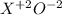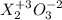If element x forms the oxides xo and x2o3 the oxidation numbers of element x are
a)+1 and +2<...

Chemistry, 21.05.2020 04:04 20jacksone
If element x forms the oxides xo and x2o3 the oxidation numbers of element x are
a)+1 and +2
b)+2 and +3
c)+1 and +3
d)+2 and +4

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
Questions

English, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50




English, 28.01.2021 18:50


Mathematics, 28.01.2021 18:50

Physics, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50


English, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50


Mathematics, 28.01.2021 18:50


Biology, 28.01.2021 18:50