
Chemistry, 19.05.2020 03:01 kayla32213
Methane, CH 4 , burns in oxygen to give carbon dioxide and water according to the following equation:
CH 4 + 2 O 2 > CO 2 + 2 H 2 O
In one experiment, a mixture of 0.250 mol of methane was burned in 1.25 mol of oxygen in a sealed steel
vessel. Find the limiting reactant and excess reactants.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1


Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2
You know the right answer?
Methane, CH 4 , burns in oxygen to give carbon dioxide and water according to the following equation...
Questions

English, 04.02.2020 05:56

Mathematics, 04.02.2020 05:56

History, 04.02.2020 05:56


Biology, 04.02.2020 05:56

Mathematics, 04.02.2020 05:56


History, 04.02.2020 05:56


Arts, 04.02.2020 05:56



Biology, 04.02.2020 05:56



Mathematics, 04.02.2020 05:56




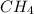 is the limiting reagent and
is the limiting reagent and 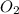 is the excess reagent.
is the excess reagent.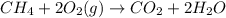
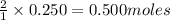 of
of 

