Chemistry, 17.05.2020 04:57 kenisonpaigebosma
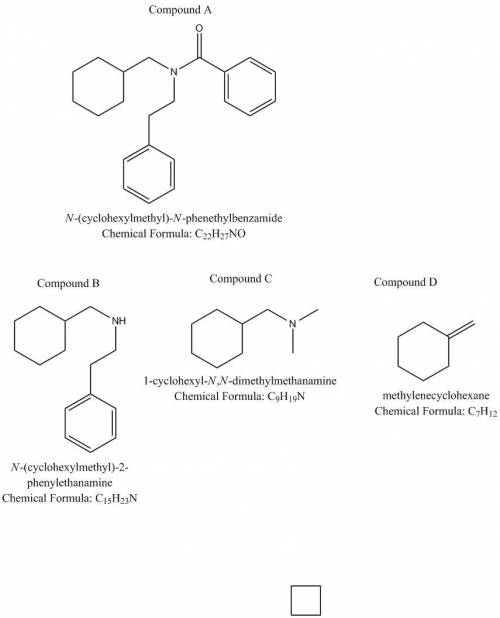
A compound A (C22H27NO) is insoluble in acid and base but reacts with concentrated aqueous HCl and heat to give a clear aqueous solution from which, on cooling, benzoic acid precipitates. When the supernatant solution is made basic, a liquid B separates. Compound B is achiral. Treatment of B with benzoyl chloride in pyridine gives back A. Evolution of gas is not observed when B is treated with an aqueous solution of NaNO2 and HCl. Treatment of B with excess CH3I, then aqueous Ag2O and heat, gives a compound C, C9H19N, plus styrene, C6H5–CH=CH2. Compound C, when treated with excess CH3I, then aqueous Ag2O and heat, gives a single alkene D that is identical to the compound obtained when cyclohexanone is treated with the ylid –:CH2– PPh3. Give the structure of A.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
A compound A (C22H27NO) is insoluble in acid and base but reacts with concentrated aqueous HCl and h...
Questions






English, 03.03.2020 06:06

Mathematics, 03.03.2020 06:06







History, 03.03.2020 06:06



History, 03.03.2020 06:06