
Chemistry, 13.05.2020 09:57 hannahrasco4051
The activation energy for proline isomerization of a peptide depends on the identity of the preceding residue and obeys Arrhenius rate behavior. Experiments are conducted on the isomerization of an alanine- proline peptide. At 25°C (298 K) the observed rate constant is 0.05 sec–1 and the value of EA is calculated to be 60 kJ•mol–1. Similar measurements are performed on a phenylalanine-proline peptide at 25°C, with a measured rate constant of 0.005 sec–1. Assuming an identical preexponential factor as the alanine-proline peptide, what is the activation energy for this peptide (kJ/mol)?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1
You know the right answer?
The activation energy for proline isomerization of a peptide depends on the identity of the precedin...
Questions

Mathematics, 08.01.2021 17:10

Mathematics, 08.01.2021 17:10

Physics, 08.01.2021 17:10



Mathematics, 08.01.2021 17:10


Computers and Technology, 08.01.2021 17:10



Mathematics, 08.01.2021 17:10



Mathematics, 08.01.2021 17:10






English, 08.01.2021 17:10

 , where k is rate constant, A is pre-exponential factor,
, where k is rate constant, A is pre-exponential factor,  is activation energy, R is gas constant and T is temperature in kelvin scale.
is activation energy, R is gas constant and T is temperature in kelvin scale.![\frac{k_{ala-pro}}{k_{phe-pro}}=e^\frac{[E_{a}^{phe-pro}-E_{a}^{ala-pro}]}{RT}](/tpl/images/0653/2427/f6d0c.png)
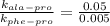 , T = 298 K , R = 8.314 J/(mol.K) and
, T = 298 K , R = 8.314 J/(mol.K) and 
![\frac{0.05}{0.005}=e^{\frac{[E_{a}^{phe-pro}-(60000J/mol)]}{8.314J.mol^{-1}.K^{-1}\times 298K}}](/tpl/images/0653/2427/26a26.png)

 (rounded off to two significant digit)
(rounded off to two significant digit)

