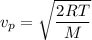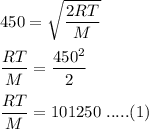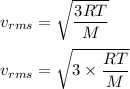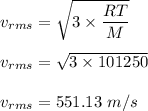Chemistry, 07.05.2020 04:58 justijust500
The most probable speed of an oxygen molecule in the gas phase at room temperature is 450 m/s. The root-mean-square speed (urms) is therefore equal to 450 m/s. much greater than 450 m/s. slightly less than 450 m/s. much less than 450 m/s. slightly greater than 450 m/s.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1


Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
The most probable speed of an oxygen molecule in the gas phase at room temperature is 450 m/s. The r...
Questions

History, 14.07.2019 21:30

Mathematics, 14.07.2019 21:30

Biology, 14.07.2019 21:30

History, 14.07.2019 21:30






Biology, 14.07.2019 21:30

History, 14.07.2019 21:30

History, 14.07.2019 21:30

Mathematics, 14.07.2019 21:30




English, 14.07.2019 21:30



History, 14.07.2019 21:30