Chemistry, 07.05.2020 05:00 tordiacasey
Consider an electrolytic cell with a platinum anode and a silver cathode in a 1.0 M AgNO3(aq) solution.
a) (3 pts) What species can be reduced in this solution? Which species is preferentially reduced? Write the reduction half- reaction. (Note that oxyanions like nitrate are not commonly reduced in aqueous electrolysis due to kinetic reasons.)
b) (2 pts) Which species is oxidized during the electrolysis? Write the oxidation half-reaction. Note that acid will form as a byproduct of the oxidation.
c) (2 pts) Write the overall electrolysis reaction in net ionic and molecular forms.
d) (2 pts) Determine the external electric potential needed for the electrolysis under standard conditions.
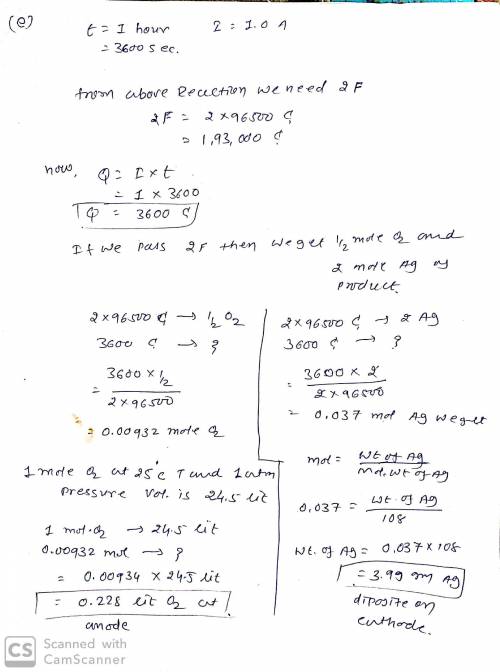
e) (5 pts) If the electrolysis is carried out for 1.00 hour using 1.00 A current, how many grams of metal will be deposited at the cathode and how many liters of gas will form at the anode at 1.00 atm pressure and 25°C?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2


Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3
You know the right answer?
Consider an electrolytic cell with a platinum anode and a silver cathode in a 1.0 M AgNO3(aq) soluti...
Questions



Mathematics, 28.09.2019 13:00






English, 28.09.2019 13:00

Social Studies, 28.09.2019 13:00

Mathematics, 28.09.2019 13:00


Biology, 28.09.2019 13:00

Mathematics, 28.09.2019 13:00




English, 28.09.2019 13:00

History, 28.09.2019 13:00