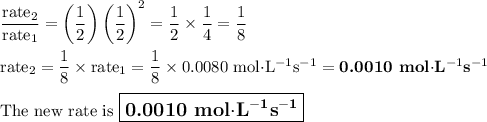The reaction is proceeding at a rate of 0.0080 Ms-1 in 50.0 mL of solution in a system with unknown concentrations of A and B. What is the rate immediately after the concentrations of all reactants are instantaneously lowered to 50% of their current value by adding an additional 50.0 mL of solvent?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
The reaction is proceeding at a rate of 0.0080 Ms-1 in 50.0 mL of solution in a system with unknown...
Questions


Chemistry, 06.08.2019 20:30



Computers and Technology, 06.08.2019 20:30



Mathematics, 06.08.2019 20:30





Physics, 06.08.2019 20:30







English, 06.08.2019 20:30

![\dfrac{\text{rate}_{2}}{\text{rate}_{1}} = \dfrac{k_{2}\text{[A]}_2[\text{B]}_{2}^{2}}{k_{1}\text{[A]}_1[\text{B]}_{1}^{2}}= \left (\dfrac{\text{[A]}_{2}}{\text{[A]}_{1}}\right ) \left (\dfrac{\text{[B]}_{2}}{\text{[B]}_{1}}\right )^{2}](/tpl/images/0651/4550/63ca5.png)
![\dfrac{\text{[A]}_{2}}{\text{[A]}_{1}} = \dfrac{1}{2}\text{ and }\dfrac{\text{[B]}_{2}}{\text{[B]}_{1}}= \dfrac{1}{2}](/tpl/images/0651/4550/c7d0e.png)