Chemistry, 07.05.2020 03:11 parrazm2022
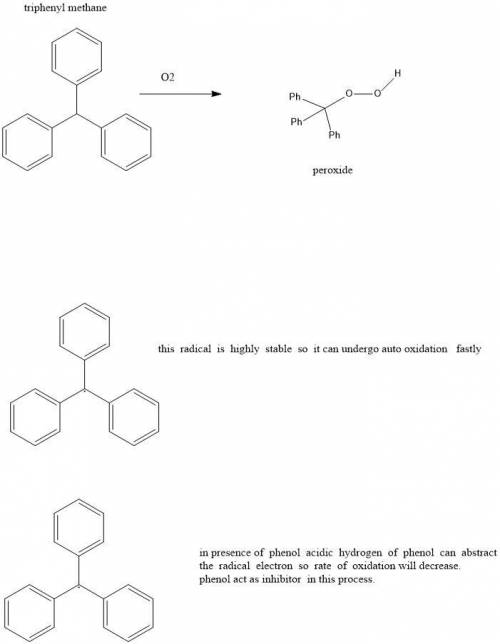
10.31 Triphenylmethane readily undergoes autooxidation to produce a hydroperoxide: c10s172 (a) Draw the expected hydroperoxide. (b) Explain why triphenylmethane is so susceptible to autooxidation. (c) In the presence of phenol (C6H5OH), triphenylmethane undergoes autooxidation at a much slower rate. Explain this observation.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
10.31 Triphenylmethane readily undergoes autooxidation to produce a hydroperoxide: c10s172 (a) Draw...
Questions

Social Studies, 26.10.2021 14:00



Mathematics, 26.10.2021 14:00

Mathematics, 26.10.2021 14:00


Computers and Technology, 26.10.2021 14:00

Chemistry, 26.10.2021 14:00



History, 26.10.2021 14:00

Biology, 26.10.2021 14:00

Mathematics, 26.10.2021 14:00

Mathematics, 26.10.2021 14:00


English, 26.10.2021 14:00

Mathematics, 26.10.2021 14:00