
Chemistry, 07.05.2020 00:58 juicemankinnie95
A 25.0 mL aliquot of 0.0430 0.0430 M EDTA EDTA was added to a 56.0 56.0 mL solution containing an unknown concentration of V 3 + V3+ . All of the V 3 + V3+ present in the solution formed a complex with EDTA EDTA , leaving an excess of EDTA EDTA in solution. This solution was back-titrated with a 0.0490 0.0490 M Ga 3 + Ga3+ solution until all of the EDTA EDTA reacted, requiring 13.0 13.0 mL of the Ga 3 + Ga3+ solution. What was the original concentration of the V 3 + V3+ solution?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
A 25.0 mL aliquot of 0.0430 0.0430 M EDTA EDTA was added to a 56.0 56.0 mL solution containing an un...
Questions

English, 22.06.2020 08:57


Geography, 22.06.2020 08:57



Mathematics, 22.06.2020 08:57



Chemistry, 22.06.2020 08:57



Mathematics, 22.06.2020 08:57

English, 22.06.2020 08:57

Biology, 22.06.2020 08:57


English, 22.06.2020 08:57



Mathematics, 22.06.2020 08:57

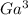 + and EDTA and
+ and EDTA and 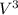 +
+
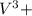 = 0.001075 - 0.00044 = 0.000675 moles
= 0.001075 - 0.00044 = 0.000675 moles


