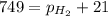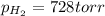Chemistry, 06.05.2020 17:08 22katelynfrankouqqrb
As the magnesium reacts, the hydrogen gas produced is collected by water displacement at 23.0oC. The pressure of the gas in the collection tube is measured to be 749 torr. Given that the equilibrium vapor pressure of water is 21 torr at 23.0oC, calculate the pressure that the H2(g) produced in the reaction would have if it were dry.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
As the magnesium reacts, the hydrogen gas produced is collected by water displacement at 23.0oC. The...
Questions

Computers and Technology, 20.09.2019 03:30

History, 20.09.2019 03:30

Mathematics, 20.09.2019 03:30


World Languages, 20.09.2019 03:30



Arts, 20.09.2019 03:30


Mathematics, 20.09.2019 03:30


Mathematics, 20.09.2019 03:30

Mathematics, 20.09.2019 03:30

Mathematics, 20.09.2019 03:30

Social Studies, 20.09.2019 03:30



Mathematics, 20.09.2019 03:30

Social Studies, 20.09.2019 03:30

Mathematics, 20.09.2019 03:30

 produced in the reaction would have if it were dry will be 728 torr
produced in the reaction would have if it were dry will be 728 torr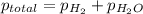
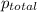 =total pressure of gas = 749 torr
=total pressure of gas = 749 torr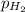 = partial pressure of hydrogen = ?
= partial pressure of hydrogen = ?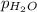 = partial pressure of water = 21 torr
= partial pressure of water = 21 torr