
Chemistry, 05.05.2020 18:40 lillianneal
Substance ΔG°f(kJ/mol) M3O4(s) −8.80 M(s) 0 O2(g) 0 Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M3O4(s)↽−−⇀ 3M(s)+2O2(g) What is the standard change in Gibbs energy for the reaction, as written, in the forward direction? ΔG∘rxn= kJ/mol What is the equilibrium constant of this reaction, as written, in the forward direction at 298 K? K= What is the equilibrium pressure of O2(g) over M(s) at 298 K?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
You know the right answer?
Substance ΔG°f(kJ/mol) M3O4(s) −8.80 M(s) 0 O2(g) 0 Consider the decomposition of a metal oxide to i...
Questions

Mathematics, 26.05.2021 14:00

Mathematics, 26.05.2021 14:00


World Languages, 26.05.2021 14:00

Physics, 26.05.2021 14:00

Chemistry, 26.05.2021 14:00


Social Studies, 26.05.2021 14:00

Health, 26.05.2021 14:00

Mathematics, 26.05.2021 14:00


History, 26.05.2021 14:00


Mathematics, 26.05.2021 14:00


Mathematics, 26.05.2021 14:00

Mathematics, 26.05.2021 14:00


Mathematics, 26.05.2021 14:00

English, 26.05.2021 14:00

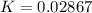
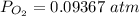
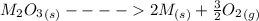
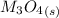 is
is 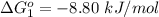
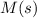 is
is 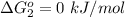
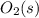 is
is 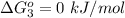
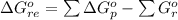
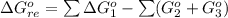
![\Delta G^o_{re} =[ (2 * 0) + (\frac{3}{2} * 0 )] - [1 * - 8.80]](/tpl/images/0641/3844/374c7.png)
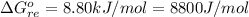

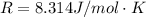
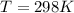
![K_p =[ P_{O_2}]^{\frac{3}{2} }](/tpl/images/0641/3844/66043.png)
![[ P_{O_2}]](/tpl/images/0641/3844/bc839.png) is the equilibrium pressure of oxygen
is the equilibrium pressure of oxygen 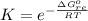
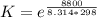
![0.02867 = [P_{O_2}]^{[\frac{3}{2} ]}](/tpl/images/0641/3844/d1a4b.png)
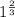
![P_{O_2} = [0.02867]^{\frac{2}{3} }](/tpl/images/0641/3844/dba69.png)


