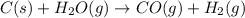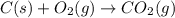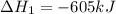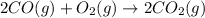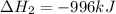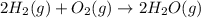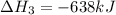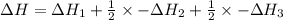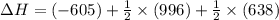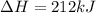QUESTION 13
Use the standard reaction enthalpies given below to determine AHºrn for the followi...

Chemistry, 05.05.2020 14:47 clarajeansonels9987
QUESTION 13
Use the standard reaction enthalpies given below to determine AHºrn for the followi
C(s) + H2O(g) - CO(g) + H2(9)
Given:
Reaction 1: C(s) + O2(g) – CO2(g) AHºrxn = -605 kJ
Reaction 2: 2 CO(g) + O2(g) – 2 CO2(g) AH°x = -966 kJ
Reaction 3: 2 H2(g) + O2(g) → 2 H2O(g) AHⓇx = -638 kJ

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
Questions

History, 29.04.2021 16:50

Mathematics, 29.04.2021 16:50

Mathematics, 29.04.2021 16:50





Mathematics, 29.04.2021 16:50

History, 29.04.2021 16:50


Mathematics, 29.04.2021 16:50


Mathematics, 29.04.2021 16:50


Mathematics, 29.04.2021 16:50



Mathematics, 29.04.2021 16:50

Social Studies, 29.04.2021 16:50

Health, 29.04.2021 16:50

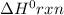 for the reaction is 212 kJ
for the reaction is 212 kJ