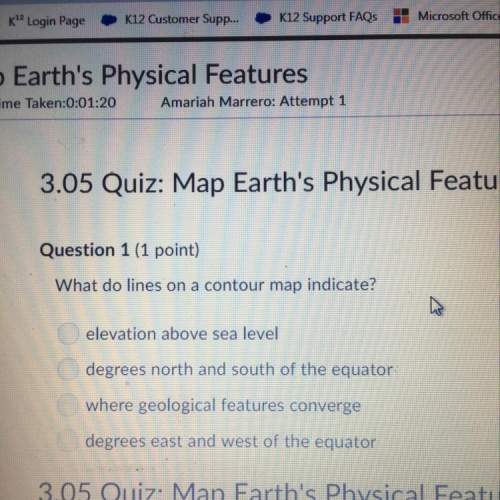
Chemistry, 04.05.2020 22:53 AnonymousLizard52303
The enthalpies of formation of the compounds in the combustion of methane,
Upper C upper H subscript 4 (g) plus 2 upper O subscript 2 (g) right arrow upper C upper o subscript 2 (g) plus 2 upper H subscript 2 upper O (g).,
are CH4 (g): Delta. Hf = –74.6 kJ/mol; CO2 (g): Delta. Hf = –393.5 kJ/mol; and H2 O(g): Delta. Hf = –241.82 kJ/mol.
How much heat is released by the combustion of 2 mol of methane?
Use Delta H r x n equals the sum of delta H f of all the products minus the sum of delta H f of all the reactants..
-80.3 kJ
-802.5 kJ
-1,605.1 kJ
-6,420.3 kJ

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3


Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1
You know the right answer?
The enthalpies of formation of the compounds in the combustion of methane,
Upper C upper...
Upper C upper...
Questions

Mathematics, 26.02.2021 17:10


Mathematics, 26.02.2021 17:10

Mathematics, 26.02.2021 17:10

English, 26.02.2021 17:10

Mathematics, 26.02.2021 17:10

Mathematics, 26.02.2021 17:10

Mathematics, 26.02.2021 17:10

History, 26.02.2021 17:10



Geography, 26.02.2021 17:10

Mathematics, 26.02.2021 17:10

Mathematics, 26.02.2021 17:10


Mathematics, 26.02.2021 17:10


Physics, 26.02.2021 17:10


Mathematics, 26.02.2021 17:10