
Chemistry, 03.05.2020 13:07 locomexicano03
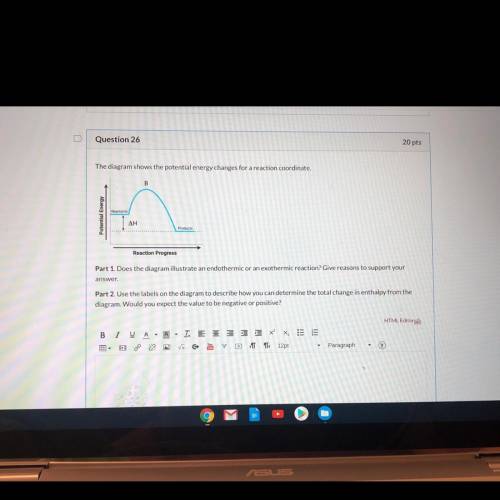
Part 1: Does the diagram illustrate an endothermic or an exothermic reaction?
Part 2: Use the labels on the diagram to describe how you can determine the total change in enthalpy from the diagram. Would you expect the value to be negative or positive?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1
You know the right answer?
Part 1: Does the diagram illustrate an endothermic or an exothermic reaction?
Part 2: Use the...
Part 2: Use the...
Questions





Mathematics, 25.07.2019 02:30

English, 25.07.2019 02:30

Mathematics, 25.07.2019 02:30

Mathematics, 25.07.2019 02:30


English, 25.07.2019 02:30

English, 25.07.2019 02:30

History, 25.07.2019 02:30

Chemistry, 25.07.2019 02:30

Social Studies, 25.07.2019 02:30








