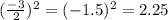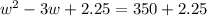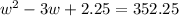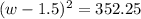Introduction: This activity introduces you to solutions and allows you to experience making different
concentrations of Kool-aid solution. There are many ways to calculate the concentration of a substance
including: molarity (M), parts per million (ppm), percent composition (% comp), and grams per liter (g/L). In
chemistry, concentration is usually measured by the number of moles of substance/ liter of substance, or
Molarity.
Materials:
• Kool-Aid Powder with SUGAR already included (or sugar if you don’t have kool-aid)
• Something to stir solutions
• 4 large cups
• measuring cup (ml preferred but cups will work)
Instructions: In this activity you will be making a series of kool-aid solutions. Make sure to record all data and
answer all the questions below.
Step 1: You will prepare a 237.5 ml kool-aid solution with a concentration of 1M. The molar mass of kool-aid
(or sugar since kool-aid is basically sugar C12H22O11.) is 342 g/mol. Show the calculations below which helped
you create your 1M solution. Note: 237.5 mL is approximately one cup.
Please show your work here.
Liters of water needed
Molarity of kool-aid needed
Grams of kool-aid needed
Now prepare the solution and make sure to label it (sticky note, pen on plastic cup, piece of paper under
cup, etc…)
To make this solution:
1. Measure approximately ½ dry cups of the Kool-aid mixture.
2. Pour the Kool-aid powder you measured into a large glass or cup.
3. Measure out the appropriate amount of water (1 cup) and mix into your glass/cup.
5. Stir to mix and label with the appropriate molarity.
6. Don’t taste test it yet, that will come later.
Do not discard the leftover 1M solution; we are not done with it yet!
Step 2: Using the 1M solution above you will create a second solution with a molarity of 0.5 M solution and
volume of 237.5 mL of kool-aid. Watch video link below for help.
Show your work here. You may need the following equation M1V1=M2V2 where M stands for molarity and V
standards for volume. :
Volume of 1M solution needed
Volume of water needed
Now prepare the solution and make sure to label it! Do not discard the leftover 1M and 0.5 M solutions;
we are not done with them yet!
Step 3: Using the 0.5 M solution above you will create 237.5 mL of a 0.25 M solution of kool-aid. You will need
the following equation M1V1=M2V2 where M stands for molarity and V standards for volume. Note: 237.5 ml is
about 1 cup. There is no video demonstration of this calculation, but follow the same dilution calculation
procedure as was demonstrated in step 2.
Show your work here:
Volume of 0.5 M solution needed:
Volume of water needed:
Now prepare the solution, make sure to label! Do not discard the leftover solutions; we are not done
with them yet!

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
Introduction: This activity introduces you to solutions and allows you to experience making differen...
Questions


Mathematics, 15.05.2020 08:57



History, 15.05.2020 08:57

Mathematics, 15.05.2020 08:57

Mathematics, 15.05.2020 08:57


Social Studies, 15.05.2020 08:57

Mathematics, 15.05.2020 08:57

Mathematics, 15.05.2020 08:57


Physics, 15.05.2020 08:57

History, 15.05.2020 08:57


History, 15.05.2020 08:57

Mathematics, 15.05.2020 08:57

Mathematics, 15.05.2020 08:57


Mathematics, 15.05.2020 08:57