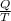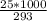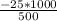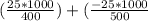Chemistry, 05.05.2020 05:40 olejlund8073
An object at 20∘C absorbs 25.0 J of heat. What is the change in entropy ΔS of the object? Express your answer numerically in joules per kelvin. ΔS = nothing J/K Request Answer Part E An object at 500 K dissipates 25.0 kJ of heat into the surroundings. What is the change in entropy ΔS of the object? Assume that the temperature of the object does not change appreciably in the process. Express your answer numerically in joules per kelvin. ΔS = nothing J/K Request Answer Part F An object at 400 K absorbs 25.0 kJ of heat from the surroundings. What is the change in entropy ΔS of the object? Assume that the temperature of the object does not change appreciably in the process. Express your answer numerically in joules per kelvin. ΔS = nothing J/K Request Answer Part G Two objects form a closed system. One object, which is at 400 K, absorbs 25.0 kJ of heat from the other object, which is at 500 K. What is the net change in entropy ΔSsys of the system? Assume that the temperatures of the objects do not change appreciably in the process. Express your answer numerically in joules per kelvin.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1



You know the right answer?
An object at 20∘C absorbs 25.0 J of heat. What is the change in entropy ΔS of the object? Express yo...
Questions



Mathematics, 21.05.2020 23:05










Mathematics, 21.05.2020 23:05






Physics, 21.05.2020 23:05