
Consider the two reduction half-reactions: Na+(aq) + e− LaTeX: \longrightarrow⟶ Na(s) Eo = −2.71 V Cl2(g) + 2 e− LaTeX: \longrightarrow⟶ 2 Cl−(aq) Eo = 1.36 VUse the electrode potentials above to calculate Eocell and ∆Gorxn for the reaction below, and determine if it is the reaction for a voltaic cell or an electrolytic cell. 2 Na+(aq) + 2 Cl−(aq) LaTeX: \longrightarrow⟶2 Na(s) + Cl2(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
Consider the two reduction half-reactions: Na+(aq) + e− LaTeX: \longrightarrow⟶ Na(s) Eo = −2.71 V C...
Questions

History, 20.03.2021 18:50




Mathematics, 20.03.2021 18:50

English, 20.03.2021 18:50

Mathematics, 20.03.2021 18:50



Mathematics, 20.03.2021 18:50



Mathematics, 20.03.2021 18:50

Mathematics, 20.03.2021 18:50

Mathematics, 20.03.2021 18:50

Chemistry, 20.03.2021 18:50

Mathematics, 20.03.2021 18:50


English, 20.03.2021 18:50

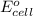 = -4.07v
= -4.07v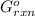 = +785.510 KJ
= +785.510 KJ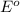 and Δ
and Δ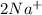 +
+ 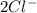 →
→ 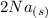 +
+ 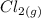
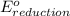 - Δ
- Δ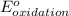 = -2.71v - 1.36v = -4.07V
= -2.71v - 1.36v = -4.07V

