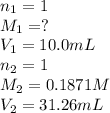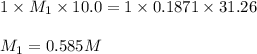G Suppose you are titrating vinegar, which is an acetic acid solution of unknown strength, with a sodium hydroxide solution according to the equation H C 2 H 3 O 2 + N a O H ⟶ H 2 O + N a C 2 H 3 O 2 If you require 31.26 mL of 0.1871 M N a O H solution to titrate 10.0 mL of H C 2 H 3 O 2 solution, what is the concentration of acetic acid in the vinegar?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
G Suppose you are titrating vinegar, which is an acetic acid solution of unknown strength, with a so...
Questions

Social Studies, 14.07.2019 23:20










Spanish, 14.07.2019 23:20

Mathematics, 14.07.2019 23:20

History, 14.07.2019 23:20



Advanced Placement (AP), 14.07.2019 23:20

World Languages, 14.07.2019 23:20



Mathematics, 14.07.2019 23:20

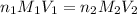
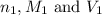 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 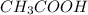
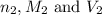 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.