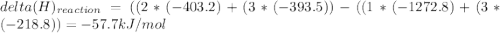Chemistry, 05.05.2020 08:30 jackiediaz
Use the given heats of formation to calculate the enthalpy change for this reaction. B2O3(g) + 3COCl2(g) →2BCl3(g) + 3CO2(g) ΔHof of B2O3(g) is –1272.8 kJ∙mol–1 ΔHof of BCl3(g) is –403.8 kJ∙mol–1 ΔHof of COCl2(g) is –218.8 kJ∙mol–1 ΔHof of CO2(g) is –393.5 kJ∙mol–1 Question 8 options: 1) 649.3 kJ·mol–1 2) 354.9 kJ·mol–1 3) –58.9 kJ·mol–1 4) –3917.3 kJ·mol–1

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
Use the given heats of formation to calculate the enthalpy change for this reaction. B2O3(g) + 3COCl...
Questions




Mathematics, 26.04.2020 12:42


Mathematics, 26.04.2020 12:43

Biology, 26.04.2020 12:43

Health, 26.04.2020 12:43



Mathematics, 26.04.2020 12:43

Mathematics, 26.04.2020 12:44



Mathematics, 26.04.2020 12:44


History, 26.04.2020 12:45