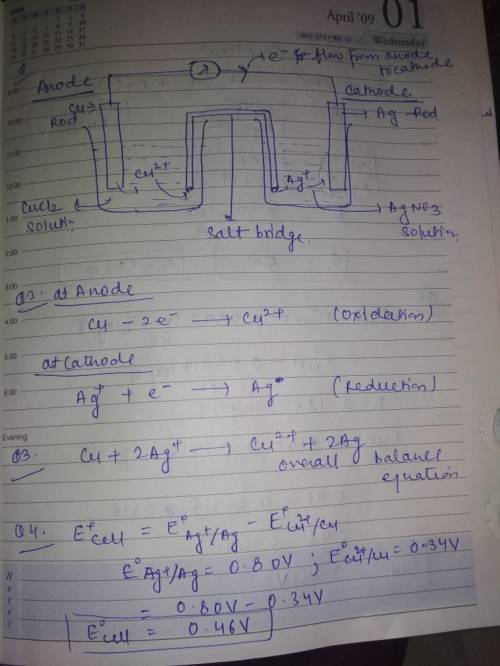
Cu(s), CuCl2 (0.50 M) || Ag(s), AgNO3 (0.010 M) 1.
Draw the schematic of the electroche...

Chemistry, 05.05.2020 09:05 jamarian101
Cu(s), CuCl2 (0.50 M) || Ag(s), AgNO3 (0.010 M) 1.
Draw the schematic of the electrochemical cell that you created including all the components (metals, solutions, salt bridges, voltmeters, etc.) in this portion of the experiment. Annotate on the schematic which side is the anode, which side is the cathode, the sign of each half cell, the composition of the metals and solutions, and the direction of the flow of the electrons through the cell.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1


Chemistry, 23.06.2019 06:00
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
You know the right answer?
Questions



Mathematics, 02.09.2020 01:01


Health, 02.09.2020 01:01

English, 02.09.2020 01:01


Mathematics, 02.09.2020 01:01

Business, 02.09.2020 01:01

Chemistry, 02.09.2020 01:01


Mathematics, 02.09.2020 01:01




Biology, 02.09.2020 01:01

Mathematics, 02.09.2020 01:01

Physics, 02.09.2020 01:01

Mathematics, 02.09.2020 01:01

History, 02.09.2020 01:01