
Chemistry, 05.05.2020 16:30 hhhhhh8897
21-B. Mn was used as an internal standard for measuring Fe by atomic absorption. A standard mixture containing 2.00 mg Mn/mL and 2.50 mg Fe/mL gave a quotient (Fe signal/Mn signal) 5 1.05/1.00. A mixture with a volume of 6.00 mL was prepared by mixing 5.00 mL of unknown Fe solution with 1.00 mL containing 13.5 mg Mn/mL. The absorbance of this mixture at the Mn wave- length was 0.128, and the absorbance at the Fe wavelength was 0.185. Find the molarity of the unknown Fe solution.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 23.06.2019 06:50
Organisms are classified as producer or consumers according to the way they ?
Answers: 1
You know the right answer?
21-B. Mn was used as an internal standard for measuring Fe by atomic absorption. A standard mixture...
Questions

Mathematics, 26.08.2019 09:50

History, 26.08.2019 09:50

Social Studies, 26.08.2019 09:50

Mathematics, 26.08.2019 09:50




Mathematics, 26.08.2019 09:50

Mathematics, 26.08.2019 09:50

Biology, 26.08.2019 09:50



Social Studies, 26.08.2019 09:50



Mathematics, 26.08.2019 09:50


Mathematics, 26.08.2019 09:50

Social Studies, 26.08.2019 09:50

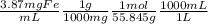 = 0.0693M Fe
= 0.0693M Fe

