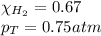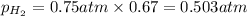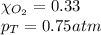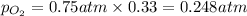Chemistry, 05.05.2020 22:21 ayoismeisjjjjuan
Solve the following, show all work and units for your calculation: Let's say we have a mixture of hydrogen gas
(H2), and oxygen gas (O2). The mixture contains 6.7 mol hydrogen gas and 3.3 mol oxygen gas. The mixture is
in a 300 L container at 273 K and the total pressure of the gas mixture is 0.75 atm. What is the partial
pressure for each gas?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
Solve the following, show all work and units for your calculation: Let's say we have a mixture of hy...
Questions

Chemistry, 03.12.2021 02:50



Mathematics, 03.12.2021 02:50





Spanish, 03.12.2021 02:50

Mathematics, 03.12.2021 02:50

Social Studies, 03.12.2021 02:50



Engineering, 03.12.2021 02:50

Mathematics, 03.12.2021 02:50

Computers and Technology, 03.12.2021 02:50


Advanced Placement (AP), 03.12.2021 02:50

Computers and Technology, 03.12.2021 02:50

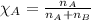
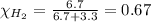
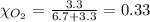
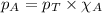
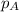 = partial pressure of substance
= partial pressure of substance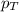 = total pressure
= total pressure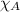 = mole fraction of substance
= mole fraction of substance