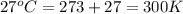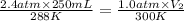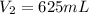Chemistry, 06.05.2020 04:32 patriciahonsakpa6u5f
A diver exhales a bubble with a volume of 250 mL at a pressure of 2.4 atm and a temperature of 15 °C. What is the volume of the bubble when it reaches the surface where the pressure is 1.0 atm and the temperature is 27 °C, if the moles are constant?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2
You know the right answer?
A diver exhales a bubble with a volume of 250 mL at a pressure of 2.4 atm and a temperature of 15 °C...
Questions


Computers and Technology, 29.01.2020 19:55

Spanish, 29.01.2020 19:55

Mathematics, 29.01.2020 19:55


Chemistry, 29.01.2020 19:55


Mathematics, 29.01.2020 19:55


Chemistry, 29.01.2020 19:55



History, 29.01.2020 19:55

Health, 29.01.2020 19:55

Chemistry, 29.01.2020 19:55




Chemistry, 29.01.2020 19:55

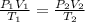
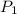 = initial pressure of gas = 2.4 atm
= initial pressure of gas = 2.4 atm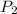 = final pressure of gas = 1.0 atm
= final pressure of gas = 1.0 atm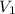 = initial volume of gas = 250 mL
= initial volume of gas = 250 mL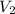 = final volume of gas = ?
= final volume of gas = ?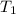 = initial temperature of gas =
= initial temperature of gas = 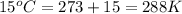
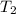 = final temperature of gas =
= final temperature of gas =