Chemistry, 06.05.2020 07:22 babyduckies37
The contents of a rock have a 206Pb to 238U mass ratio of 0.135:1.00. Assuming that the rock did not contain any 206Pb at the time of its formation, determine the age of the rock. Uranium-238 decays to lead-206 with a half-life of 4.5 billion years. Express the time to two significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1


Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
The contents of a rock have a 206Pb to 238U mass ratio of 0.135:1.00. Assuming that the rock did not...
Questions


Biology, 13.12.2019 19:31


Spanish, 13.12.2019 19:31

Mathematics, 13.12.2019 19:31


History, 13.12.2019 19:31


English, 13.12.2019 19:31




English, 13.12.2019 19:31






Health, 13.12.2019 19:31

English, 13.12.2019 19:31

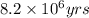
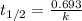
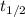 = half life of the reaction = 4.5 billion years =
= half life of the reaction = 4.5 billion years = 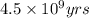
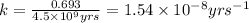
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0647/7147/f1041.png)
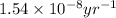
![[A_o]](/tpl/images/0647/7147/dc622.png) = initial amount of the sample = [1.00 + 0.135] = 1.135 g
= initial amount of the sample = [1.00 + 0.135] = 1.135 g