
Chemistry, 06.05.2020 06:58 LarryJoeseph
One type of breathalyzer employs a fuel cell to measure the quantity of alcohol in the breath. When a suspect blows into the breathalyzer, ethyl alcohol is oxidized to acetic acid at the anode:
CH3CH2OH(g)+4OH−(aq)→HC2H3O2(g)+3H2 O(l)+4e−
At the cathode, oxygen is reduced:
O2(g)+2H2O(l)+4e−→4OH−(aq)
The overall reaction is the oxidation of ethyl alcohol to acetic acid and water. When a suspected drunk driver blows 186 mL of his breath through this breathalyzer, the breathalyzer produces an average of 320 mA of current for 10 s.
Required:
Assuming a pressure of 1.0 atm and a temperature of 26C, what percent (by volume) of the driver's breath is ethanol?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1


Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2
You know the right answer?
One type of breathalyzer employs a fuel cell to measure the quantity of alcohol in the breath. When...
Questions


Computers and Technology, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00


Mathematics, 21.09.2021 14:00


English, 21.09.2021 14:00

English, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00

English, 21.09.2021 14:00



Mathematics, 21.09.2021 14:00




Social Studies, 21.09.2021 14:00


History, 21.09.2021 14:00

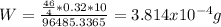
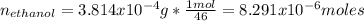
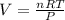
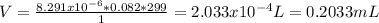
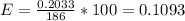 %
%

