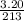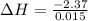Chemistry, 23.04.2020 02:56 LindaCat78
A "coffee-cup" calorimetry experiment is run for the dissolution of 3.20 g of aluminum nitrate placed into 103.2 mL of water. The temperature of the solution is initially at 23.2 oC. After the reaction takes place, the temperature of the solution is 17.7 oC. How much heat was absorbed or lost by the surroundings? Use 4.184 J/goC for the specific heat of the solution. Put your answer in units of kJ and make sure the sign is correct. What would be the enthalpy for the dissolution reaction of one mole of aluminum nitrate? Put your answer in kJ/mol and watch the sign for the enthalpy.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:20
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3


Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
A "coffee-cup" calorimetry experiment is run for the dissolution of 3.20 g of aluminum nitrate place...
Questions

Mathematics, 15.02.2021 04:30

Mathematics, 15.02.2021 04:30

Computers and Technology, 15.02.2021 04:30


Mathematics, 15.02.2021 04:30

English, 15.02.2021 04:30




Mathematics, 15.02.2021 04:30

Mathematics, 15.02.2021 04:30


History, 15.02.2021 04:30

Mathematics, 15.02.2021 04:30

Mathematics, 15.02.2021 04:30

Mathematics, 15.02.2021 04:30


Mathematics, 15.02.2021 04:30


Mathematics, 15.02.2021 04:30

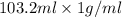
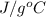 .
.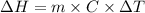
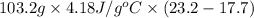
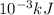 . So, 2372.5 J will be converted into kJ as follows.
. So, 2372.5 J will be converted into kJ as follows.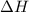 = 2.37 kJ
= 2.37 kJ 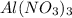 = 213 g/mol
= 213 g/mol